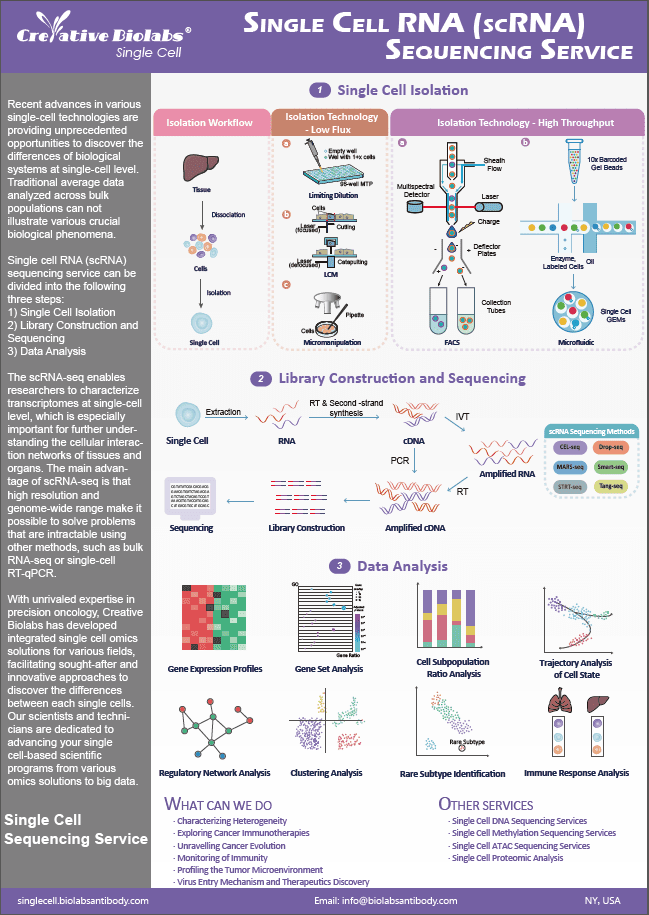
Stable Cell Line Development Services
Stable Cell Line Development Services
Creative Biolabs is proud to announce stable cell line development services for biopharmaceutical as well as research applications. The greatest benefit of employing stable cell lines in cell-based assays and cell-derived productions is overcoming the variability in reproducibility of the gene of interest expression level. It provides a stable, reproducible, and enduring expression of the gene of interest.
Numerous drug discovery assays, including screening and validation, rely on stably transfected cell lines. Using a stable cell line eliminates numerous production issues and inconsistencies associated with hybridoma production or standard transient expression, as compared with conventional antibody production techniques. Using a stable cell line to express the gene of interest allows for cost-effective large-scale production. A stable cell line enables the persistent and reproducible expression of your target gene over multiple passages.
 Fig.1 Schematic representation of stable CHO-DG44 cell line development.1.3
Fig.1 Schematic representation of stable CHO-DG44 cell line development.1.3
Published Data
| Paper Title | Generation and characterization of a stable cell line persistently replicating and secreting the human hepatitis delta virus |
| Journal | Scientific Reports |
| Published | 2019 |
| Abstract | Human HDV (hepatitis delta virus) causes the worst viral hepatitis, infecting 15–25 million people chronically. HDV uses human HBV (hepatitis B virus)-encoded envelope proteins to exit and enter hepatocytes as a satellite virus. HDV particles can only be produced in vitro by co-transfecting cells with HDV/HBV cDNAs. This method is limited. We created HuH7-END cells that continuously release infectious HDV virions. Stepwise stable integration of the HDV antigenome cDNA, HBV envelope protein genes, and HBV/HDV receptor sodium taurocholate co-transporting polypeptide (NTCP) gene produced the cell line. HuH7-END cells release 400 million HDV particles/milliliter and spread the virus to co-cultured cells. NTCP expression makes HuH7-END cells susceptible to de novo HDV entry. Virus production can be scaled up for large HDV virus stocks. Finally, HuH7-END cells can be applied for screening HDV-targeting antivirals, serving as a novel HDV replication in vitro tool. |
| Result |
Two widely used human hepatic cell lines, HuH7 and HepG2, were transfected with pJC126, a plasmid containing a 1.1-fold cDNA copy of the HDV antigenome and a neomycin resistance gene, in order to create cell lines that allow steady intracellular replication of HDV. Following G418 selection, the pool of cell clones was increased and subjected to HDAg-specific immunofluorescence analysis. They observed intracellular HDAg expression in the intermediate cell lines while stepwise engineering the HuH7-END cells. A range of 18 to 55% of the cells displayed HDAg staining. It's interesting to note that only about 30% of the HuH7-END cell clone's cells strongly expressed HDAg after clonal isolation.
|
Creative Biolabs' Stable Cell Line Development Service
Creative Biolabs provides high-quality and cost-effective services for creating a cell line expressing your gene of interest with a fast turnaround time. Alongside our extensive collection of gene expression vectors, our expertise in cell culture allows us to support and simplify your research projects according to your specific needs.
 Fig.3 Workflow of Creative Biolabs' stable cell line development service. (Creative Biolabs)
Fig.3 Workflow of Creative Biolabs' stable cell line development service. (Creative Biolabs)
Our established and streamlined process offers lentiviral vector generation, lentivirus packing, and clone selection, along with gene expression evaluation QC via qPCR and western blot. For any requirements, please contact us.
Features & Benefits
-
High Yield and Consistency
Our cell lines are engineered to produce high and consistent levels of target therapeutic proteins, ensuring reliable and reproducible results for your research and development projects.
-
High-Performance Media and Process Design
The integration of proprietary media and upstream process design ensures optimal performance of the developed cell lines, supporting high product yield and quality.
-
Robust Genetic Stabilit
The use of advanced genetic engineering techniques ensures the long-term stability of the cell lines. This genetic stability is crucial for maintaining consistent protein expression levels over extended periods, which is essential for reliable biomanufacturing.
-
Versatile Expression Systems
We offer a variety of expression systems, including inducible and non-inducible promoters, to accommodate different protein expression needs. This versatility allows for the expression of a wide range of proteins.
Q&As
Q: How long does the stable cell line development process take?
A: The timeline can vary, but our advanced technologies and streamlined processes typically shorten development times. On average, it takes around 6 to 12 months to develop a stable cell line ready for cGMP manufacturing.
Q: What factors influence the choice of host cell line?
A: The choice of host cell line is influenced by factors such as the nature of the protein being produced, the required post-translational modifications, production yield, and scalability. CHO cells are often preferred for their versatility and regulatory acceptance.
Q: What quality control measures are in place for stable cell lines?
A: Quality control measures include genetic stability testing, protein expression analysis, and thorough characterization of the cell lines. This ensures that the cell lines meet the required standards for biopharmaceutical production.
Q: How do you ensure high productivity in stable cell lines?
A: High productivity is ensured through the use of advanced gene expression systems,which optimize gene integration and expression levels. Rigorous screening and selection processes further enhance productivity.
Q: How do you select high-producing clones?
A: High-producing clones are selected using high-throughput screening technologies that assess productivity, growth characteristics, and stability. Techniques like fluorescence-activated cell sorting (FACS) and automated imaging ensure accurate and efficient selection of the best-performing clones.
Resources
References
- Toktay, Yagmur et al. "Engineering and validation of a dual luciferase reporter system for quantitative and systematic assessment of regulatory sequences in Chinese hamster ovary cells." Scientific reports vol. 12,1 6050. 11 Apr. 2022, doi:10.1038/s41598-022-09887-2.
- Ni, Yi et al. "Generation and characterization of a stable cell line persistently replicating and secreting the human hepatitis delta virus." Scientific reports vol. 9,1 10021. 10 Jul. 2019, doi:10.1038/s41598-019-46493-1.
- Distributed under Open Access license CC BY 4.0, without modification.
Search...


 Fig.2 Establishment and characterization of HDV replicating cell lines.2.3
Fig.2 Establishment and characterization of HDV replicating cell lines.2.3