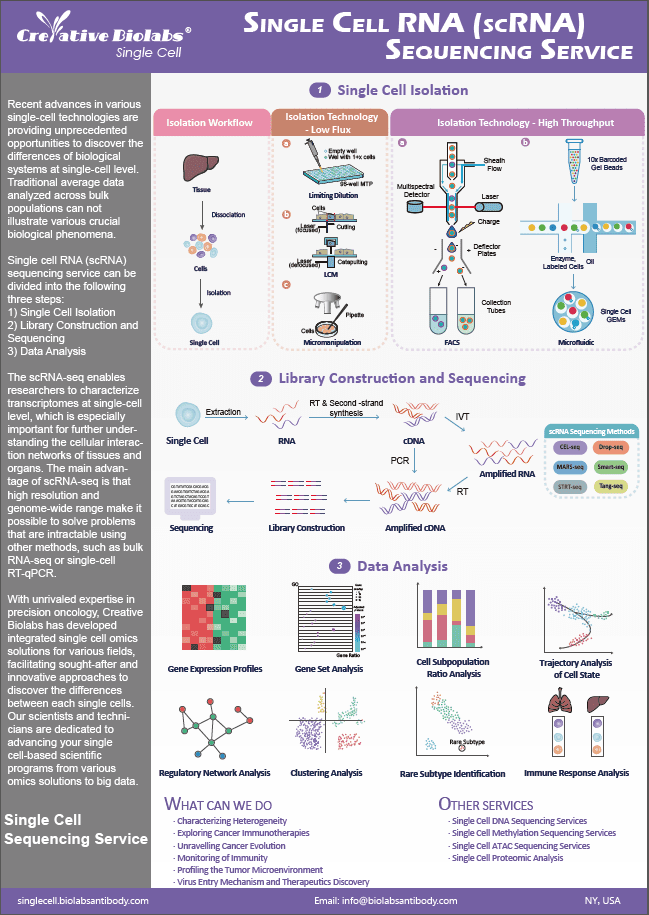
Single Cell Mass Cytometry Service
CyTOF uses antibodies with metal isotope labels to label cells and analyzes the composition of labels on each cell by time-of-flight mass spectrometry to conduct in-depth research on cell phenotype and function.
The working principle of CyTOF is analogous to that of FFC (Fluorescence Flow Cytometry), as both rely on binding target proteins with differently labeled antibodies for data generation. The only difference in CyTOF is that a pure heavy metal ion tag is applied in place of fluorochrome, and then it is subjected to mass spectrometry for data generation.
Advantages of CyTOF
- A high-throughput tool that can measure more than 40 parameters per cell.
- Low background signal.
- Suitable for analysis of cell-surface and cytoplasmic proteins.
- Applicable to fixed-cell analysis.
- Mature data analytics.
Limitations of CyTOF
- Typically requires a large sample size, owing to low recovery.
- Not suitable for live-cell analysis.
- Limited availability of metal-isotope-labeled antibodies.
The CyTOF Technical Workflow
- Tagging antibodies with stable, non-radioactive rare-earth-metal isotopes.
- Cells are immunostained with these tagged antibodies.
- Each labeled cell is then nebulized into a droplet and introduced into an argon plasma.
- The droplets containing single cells are then vaporized and the cellular components in the droplet are ionized.
- The ionized sample is passed into a mass filter, which allows only the heavy receptor ions through.
- These ions are then analyzed by a time-of-flight mass spectrometer.
Creative Biolabs has methodically developed every stage of the process, from sample preparation through data analysis, to assure high-quality study outcomes. If you have any requirements, please feel free to contact us for further communication about your project.
Q&As
Q: How does CyTOF differ from traditional flow cytometry?
A: Unlike traditional flow cytometry that uses fluorochrome-labeled antibodies, CyTOF utilizes antibodies tagged with pure heavy metal ions, reducing signal overlap and background noise, thus improving the analysis clarity.
Q: What kind of samples can be analyzed with CyTOF?
A: CyTOF can analyze various types of clinical samples, including peripheral blood and tissue suspensions. Both fresh and cryopreserved samples can be processed.
Q: Can CyTOF be used to track cell interaction with pathogens?
A: Yes, CyTOF has been successfully used to track immune cell interactions with pathogens, such as bacteria in models of infection, highlighting its utility in understanding infectious diseases and host-pathogen dynamics.
Q: Is CyTOF suitable for research on infectious diseases?
A: Absolutely, CyTOF excels in infectious disease research by enabling the high-dimensional analysis of immune responses to pathogens. It has been used to map immune dynamics in response to infections like COVID-19, aiding in the development of targeted treatments.
Q: What support is available for CyTOF users?
A: Technical support for CyTOF users includes comprehensive user manuals, online tutorials, and customer service teams that assist with instrument operation, troubleshooting, and data analysis, ensuring users can maximize the potential of this powerful technology.
Resources
Search...