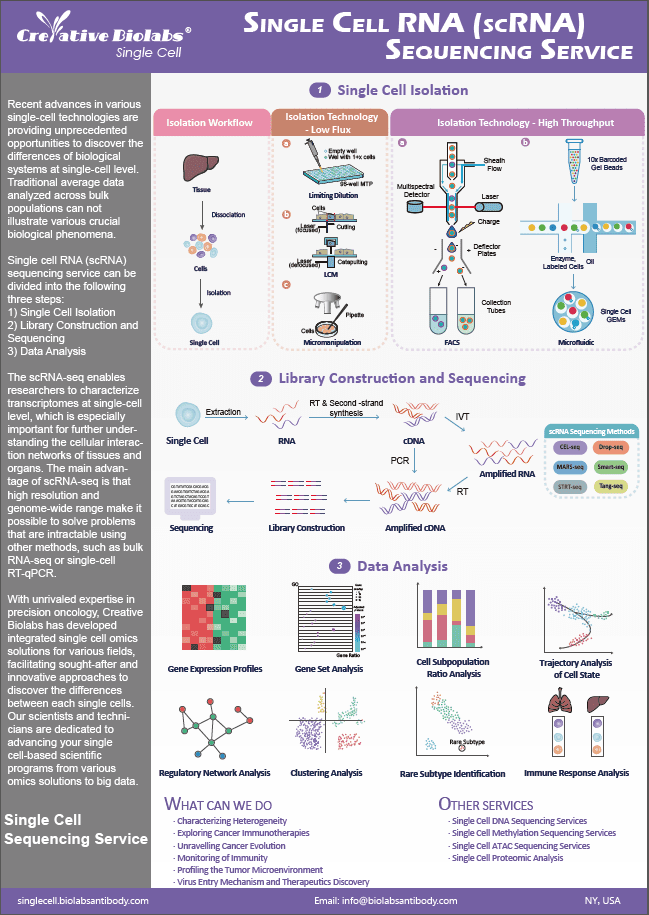
- Home
- Single Cell Featured Services
- Single Cell Functional Analysis Services
- Single Cell Death Analysis
Single Cell Death Analysis Service
Creative Biolabs has accumulated rich experience in the field of single-cell to help you plan and optimize your single cell death analysis experiments.
Cell Death
Cell death plays an essential role in the development of organisms, the etiology of disease, and the reactions of cells to therapeutic treatments. Apoptosis is a type of programmed cell death that involves caspases, which are specialized cysteine proteases found as inactive proenzymes in animal cells. From the standpoint of basic research, apoptosis is a classic systems-level challenge in which complicated circuits comprising graded and competing chemical signals dictate binary life-death decisions at the single-cell level. From a clinical standpoint, diseases like cancer cause a break in the natural balance between cell proliferation and cell death, and anticancer medications are thought to work by inducing apoptosis in cancer cells.
Advantages of Our Service
- Visualize and quantify single cell death using imaging
- Automatic analysis of the time course of cell death.
- Single cell death analysis by flow cytometry
- Multiplex assessments of proliferation and apoptosis
Published Data
| Paper Title | Time-resolved, single-cell analysis of induced and programmed cell death via non-invasive propidium iodide and counterstain perfusion |
| Journal | Scientific reports |
| Published | 2016 |
| Abstract | Prior to analysis, traditional propidium iodide (PI) staining necessitates the completion of many stages, which could alter assay results as well as cell viability. In this study, through live-cell imaging during perfusion with 0.1M PI inside a microfluidic culture device, this multistep analytical method was changed into a single-step, non-toxic, real-time method. The use of dynamic PI staining as a live/dead analytical method yielded consistent results for single-cell death caused by direct or indirect stimuli. |
| Results |
They demonstrated a one-step, non-invasive, dynamic PI staining approach inside a microfluidic culture device that allowed for real-time monitoring of cell death events in prokaryotic and eukaryotic cells. To simplify real-time testing for survivor cells or to get further information on cell health, non-toxic counterstains (CALv, CALg, and PO-PRO-1) were chosen. Over the course of the experiment, the continual supply of fluorochromes in media ensured excellent distribution in dense cell cultures. As a result, appropriate fluorochrome concentrations in the M or smaller range might be achieved, minimizing non-specific cell coloring caused by fluorochrome uptake at large concentrations.
|
Creative Biolabs is a trusted partner to offer custom single cell death analysis services for our global customers. Please contact us for more information. Our experts will help design an optimal solution for your project and trouble-shoot for you throughout the whole process.
Q&As
Q: What makes Single Cell Death Analysis more sensitive and accurate than traditional methods?
A: This service uses advanced imaging and labeling techniques, providing high sensitivity and accuracy. Automated analysis tools allow for precise quantification of cell death events, enhancing data reliability and reducing human error.
Q: How is Single Cell Death Analysis useful in drug discovery?
A: In drug discovery, this analysis helps in evaluating the cytotoxic effects of new compounds on individual cells. It provides detailed insights into the efficacy and safety of potential drugs by assessing their impact on cell viability and death.
Q: How does this service handle data from heterogeneous cell populations?
A: The service employs advanced computational tools to analyze data from heterogeneous cell populations. These tools can distinguish between different cell types and their respective death pathways, providing a comprehensive understanding of cell death in mixed cell populations.
Q: How does Single Cell Death Analysis aid in understanding neurodegenerative diseases?
A: Single Cell Death Analysis helps in identifying specific neuronal cell death pathways involved in neurodegenerative diseases like Alzheimer's and Parkinson's. By analyzing how individual neurons die, researchers can develop targeted therapies to prevent or slow down these diseases.
Resources
Reference
- Krämer, Christina E M et al. "Time-resolved, single-cell analysis of induced and programmed cell death via non-invasive propidium iodide and counterstain perfusion." Scientific reports vol. 6 32104. 1 Sep. 2016, doi:10.1038/srep32104. Distributed under Open Access license CC BY 4.0, without modification.
Search...


 Fig.1 Determination of optimal propidium iodide concentration.1
Fig.1 Determination of optimal propidium iodide concentration.1