- Home
- Single Cell Research Area
- Single Cell Omics Solutions for Immunity and Microbiome
- Single Cell Omics Solutions for Immune Cells
- Single Cell Omics Solutions for Dendritic Cells (DCs)
Single Cell Omics Solutions for Dendritic Cells (DCs)
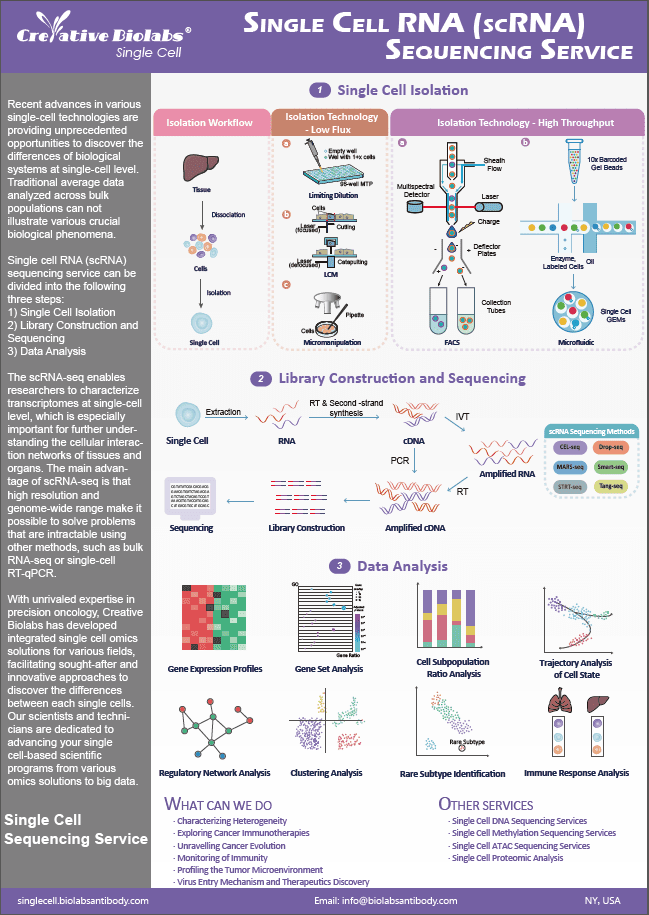
Creative Biolabs is one of the world's leading providers of single-cell technologies, including single-cell DNA sequencing (scDNA-seq), single-cell RNA-sequencing (scRNA-seq), single-cell methylation sequencing (scMethyl-Seq), single-cell proteomic analysis etc. With a wealth of experience and expertise in the field of single cell analysis, we offer comprehensive single-cell omics solutions and technical services to our customers. These comprehensive services are designed to be one-stop and we can give the results you need to advance your biological and biomedical research.
Overview of Single Cell Omics in Dendritic Cells (DCs)
DCs are specialized antigen-presenting cells that initiate T cell-mediated immune responses and function as key mediators of immunity and tolerance. DCs are heterogeneous and consist of multiple subtypes. Numerous studies have shown that human DC subtypes in the blood include CD11c+ conventional DCs (cDCs), consisting of either CD141+ or CD1c+ cells, and plasmacytoid DCs (pDCs). However, it is unclear how many DC subtypes exist, how they are related to each other and how they differ from other mononuclear phagocytes. The recent emergence of single-cell omics technologies, especially scRNA-seq, affords a direct means of identifying and fully characterizing functionally important subsets of DCs and their complex underlying biology roles. Most importantly, scRNA-seq is used to characterize and study heterogeneity across cell types, development, and dynamic processes in the immune system.
Our Single Cell Omics Solutions for Dendritic Cells (DCs)
Single-cell analysis has become a powerful method to reveal cell heterogeneity, subpopulation diversity, and rare cell characterization etc. At Single Cell, we provide comprehensive single cell omics solutions including scDNA-seq, scRNA-seq, scMethyl-Seq, scProteomic analysis to profile DCs, a critical role in immune system. In addition, our experts can provide customized single-cell omics solutions tailored to fit your specific needs at single-cell level. From our single cell omics solutions, you can identify novel cell types and characterize the cellular composition or heterogeneity in healthy and diseased tissues. We bring creative minds together for a common goal: accelerating the progress of our clients' programs within the desired timeline and budgets.
We are dedicated to providing a full spectrum of single cell analysis for DCs. Here are 2 examples using scRNA-seq technology to characterize the heterogeneity of DCs for your reference.
Example 1: Revealing the heterogeneity of pre-cDC by scRNA-seq
In humans, cDCs exist in two distinct populations characterized by expression of CD1c and CD141. cDCs arise from bone marrow (BM) progenitors that include the common DC progenitor that differentiates into the pre-cDC, the direct precursor of cDCs. Although pre-cDC has the potential to differentiate into CD1c+ and CD141+ cDCs, most pre-cDCs appear to be committed to differentiation into one or the other subset. To determine if there are two distinct precursor populations of differentially regulated cDCs in humans that are committed to either the CD1c+ or CD141+ cDC fate, Gaëlle et al. studied the transcriptomic characteristics of single pre-cDCs by scRNA-seq, and the results showed that human pre-cDCs are heterogeneous, consisting of two different precursor populations that are precommitted to become either CD1c+ or CD141+ cDCs. The two groups of lineage-primed precursors can be distinguished according to the differential expression of CD172a. Both subpopulations of pre-cDC appear in adult BM and can be found in cord blood and adult peripheral blood.
Example 2: Identification of DC subtypes by scRNA-seq
DCs and monocytes play central roles in pathogen sensing, phagocytosis and antigen presentation, and are composed of a variety of specialized subtypes. However, their identities and interrelationships are not fully understood. Villani et al. isolated approximately 2,400 cells from healthy individuals and subjected them to scRNA-seq with follow-up profiling and functional and phenotypic characterization. Using the scRNA-seq of 2,400 cells, they identified six DCs and four monocyte subtypes in human blood. They revealed a new subset of DCs that shares properties with pDCs, thus redefining pDC; a new subdivision within the CD1c+ subset of DCs; the relationship between blastic plasmacytoid DC neoplasia cells and healthy DCs; and circulating progenitor of cDCs. The revised taxonomy will enable more accurate functional and developmental analysis and immune surveillance in health and disease.
Highlight Features
- The latest single-cell isolation and sequencing technologies
- Experienced staff to assist you with program design
- Fast turnaround time
- Accurate bioinformatics analysis
- Technical support to assist with data interpretation
With senior scientists and more than 10 years' CRO services, Creative Biolabs is committed to facilitating the success of your single cell programs leveraging our comprehensive and advanced single cell multiomics platforms. For more details, please feel free to contact us.
Q&As
Q: Can single-cell omics resolve the different subtypes of DCs?
A: Yes, single-cell omics can distinguish between various DC subtypes, such as conventional DCs (cDCs) and plasmacytoid DCs (pDCs), based on their gene expression profiles. This resolution enhances our understanding of their specific roles in immune responses and pathological conditions.
Q: How does single-cell omics contribute to personalized medicine for diseases involving DCs?
A: By detailing the cellular makeup and function of DCs in individual patients, single-cell omics can guide personalized immunotherapy strategies, optimize vaccine development, and improve diagnostic accuracy, making treatments more effective and tailored to individual immune profiles.
Q: How can single-cell omics of DCs aid in the discovery of new immunotherapies?
A: By delineating the roles and interactions of various DC subsets within the immune system, single-cell omics enables the identification of novel therapeutic targets. For example, specific DC subsets that regulate immune tolerance or inflammation can be targeted to enhance or suppress immune responses, which is crucial for the treatment of autoimmune diseases or cancer.
Q: What are the benefits of studying DCs using single-cell RNA sequencing in comparison to traditional bulk RNA sequencing?
A: Single-cell RNA sequencing (scRNA-seq) provides a much higher resolution of cellular differences and a more precise understanding of cellular functions than bulk sequencing. For DCs, scRNA-seq allows researchers to uncover rare subpopulations and transient states that might be missed in bulk analyses, offering insights into their development, differentiation, and functional states within tissues.
Q: How are data from single-cell omics of DCs integrated with other data types?
A: Integration with other omics data, such as proteomics and metabolomics, is crucial for a holistic understanding of DC functions. This multi-omics approach can uncover how different molecular layers coordinate to drive DC behavior in immune responses.
Resources
Search...